Port: Shanghhai
Minimum Order Quantity:100,000 Piece/Pieces
Supply Ability: 1,000,000 Piece/Pieces per Week
Payment Terms: L/C,T/T
Packaging & Delivery
Packaging Details: 100 tests/box or as request
Delivery Detail: 3 ~ 4 weeks
Specifications
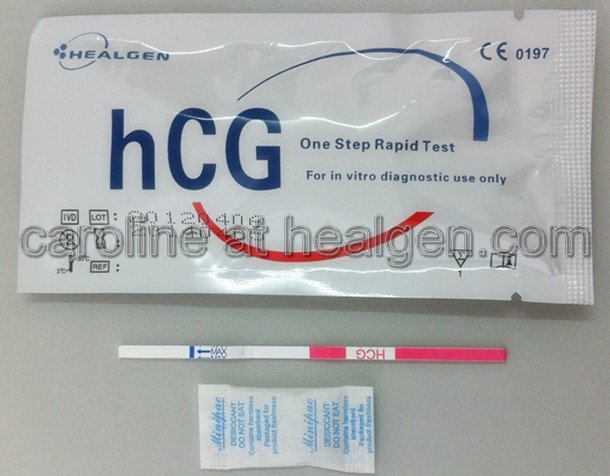
Urine Pregnancy Test Strip
FDA 510k Approval
CE Certificate (CE 0197)
ISO 13485 Certificate
Intended Use
The Urine Pregnancy Test Strip is an immunochromatographic assay designed for the rapid determination of human chorionic gonadotropin (hCG) in urine. The test is used to obtain a visual qualitative result. For in vitro self-testing use only.
Invalid
The result is invalid if no red line appears in the control region, even if a line appears in the test region. You should repeat the test with a new midstream test.
Material Provided
Each test contains:
1. One test strip
2. One desiccant
Each kit contains:
1. 100 test strips
2. 1 instruction
Minimum Order Quantity:100,000 Piece/Pieces
Supply Ability: 1,000,000 Piece/Pieces per Week
Payment Terms: L/C,T/T
Packaging & Delivery
Packaging Details: 100 tests/box or as request
Delivery Detail: 3 ~ 4 weeks
Specifications
Urine Pregnancy Test Strip
FDA 510k Approval
CE Certificate (CE 0197)
ISO 13485 Certificate
Intended Use
The Urine Pregnancy Test Strip is an immunochromatographic assay designed for the rapid determination of human chorionic gonadotropin (hCG) in urine. The test is used to obtain a visual qualitative result. For in vitro self-testing use only.
Invalid
The result is invalid if no red line appears in the control region, even if a line appears in the test region. You should repeat the test with a new midstream test.
Material Provided
Each test contains:
1. One test strip
2. One desiccant
Each kit contains:
1. 100 test strips
2. 1 instruction
Certificate
- CE
- ISO 13485
- FDA
Main Products
Pregnancy&Fertility,Infectious Diseases,drug of abuse rapid test kits
