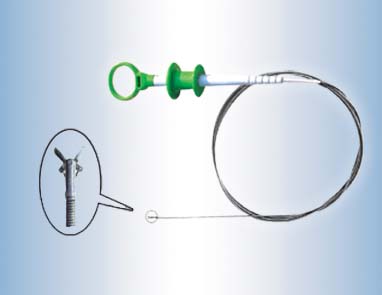
Transmed (China) Co.,Ltd registered in the PuKou economic development zone of NanJing which is specialized in the endoscopes sterilized disposable biopsy forceps .
We have our own patents; our products adopt the latest hanging hook structure, and all the components are made up of the stainless steel except the handle to avoid rust.
Transmed has powerful technical research and development team with market orientation, keeps close collaboration with the clinical expert .We developed the endoscopes sterilized disposable biopsy forceps through absorbing the suggestion of clinical expert, and also guided by the experts and professors of Wuhan University of Science & Technology.
The Normal gastroscope biopsy operation, the biopsy forceps insert into the mucousmembrane of digestive duct, and in directly contact with blood .So for the reusable biopsy forceps if sterilization is not strictly which contains the Potential risk of cross infection, increase the possibility of patients catch the hepatitis B virus and HIV .In America and Canada and French have forced by law to use the disposable biopsy forceps in the endoscopes biopsy operation .In the other countries of European and Japan the disposable biopsy forceps have been widely accepted by doctors and patients and it is going to become a trend .In 1st Jan 2004 the health department of China promulgated the law whichstrictly stated that the biopsy forceps must be sterilized, at current most Hospital have sterilized the biopsy forceps beforeusing. Normally the preparation time for 30-biopsy forceps sterilization is 2 hours And still need three Hours for ethylene epoxide if use the 2% aldehyde there need 10 hours .So the endoscope disposable biopsy forceps developed by Transmed totally meet the sanitarystandard, One patients apply for one product which completely prevent the crossing infection.
We have our own patents; our products adopt the latest hanging hook structure, and all the components are made up of the stainless steel except the handle to avoid rust.
Transmed has powerful technical research and development team with market orientation, keeps close collaboration with the clinical expert .We developed the endoscopes sterilized disposable biopsy forceps through absorbing the suggestion of clinical expert, and also guided by the experts and professors of Wuhan University of Science & Technology.
The Normal gastroscope biopsy operation, the biopsy forceps insert into the mucousmembrane of digestive duct, and in directly contact with blood .So for the reusable biopsy forceps if sterilization is not strictly which contains the Potential risk of cross infection, increase the possibility of patients catch the hepatitis B virus and HIV .In America and Canada and French have forced by law to use the disposable biopsy forceps in the endoscopes biopsy operation .In the other countries of European and Japan the disposable biopsy forceps have been widely accepted by doctors and patients and it is going to become a trend .In 1st Jan 2004 the health department of China promulgated the law whichstrictly stated that the biopsy forceps must be sterilized, at current most Hospital have sterilized the biopsy forceps beforeusing. Normally the preparation time for 30-biopsy forceps sterilization is 2 hours And still need three Hours for ethylene epoxide if use the 2% aldehyde there need 10 hours .So the endoscope disposable biopsy forceps developed by Transmed totally meet the sanitarystandard, One patients apply for one product which completely prevent the crossing infection.
Features
- single-used
- single-used
Main Products
disposable biopsy forceps
